Carestream Health’s Ultrasound Systems Receive FDA 510(k) Clearance; Now Available for Order in U.S.
Prospective Customers Praise System’s Sealed, All-Touch Control Panel, Advanced Imaging Capabilities
ROCHESTER, N.Y., June 2 — Two CARESTREAM Touch Ultrasound Systems have received FDA 510(k) Clearance and are now available for order in the United States: the CARESTREAM Touch Prime Ultrasound System and the CARESTREAM Touch Prime XE Ultrasound System. Both premium systems are designed for general diagnostic imaging use in radiology.
These two products are the first in Carestream’s new family of ultrasound systems, which earned praise for its all-touch control panel and advanced imaging capabilities when demonstrated to attendees of the 2014 Radiological Society of North America conference and the 2015 American Institute of Ultrasound in Medicine meeting.
 “These new ultrasound systems build upon our proven expertise in digital X-ray systems,” said Diana L. Nole, President, Digital Medical Solutions, Carestream. “We started with a clean slate and worked with customers to develop a new generation of ultrasound systems that enhance ease of use and productivity, while incorporating integrated GPU processing power that delivers fast response times and extremely high image quality.”
“These new ultrasound systems build upon our proven expertise in digital X-ray systems,” said Diana L. Nole, President, Digital Medical Solutions, Carestream. “We started with a clean slate and worked with customers to develop a new generation of ultrasound systems that enhance ease of use and productivity, while incorporating integrated GPU processing power that delivers fast response times and extremely high image quality.”
The CARESTREAM Touch Prime XE is the company’s top-of-the-line offering. It employs Carestream’s Touch Prime SynTek Architecture, which is a combination of breakthrough technologies that simultaneously provide enhanced spatial detail with increased frame rates for improved visualization of moving structures while optimizing image formation to reduce noise and artifacts. Imaging and Doppler improvements allow for more consistent visualization of subtle tissue contrast differences and can improve the ability to see small structures.
The Touch Prime XE is capable of frame rates in excess of 100Hz while maintaining improved imaging detail, and includes optional features such as DICOM, wireless connectivity, barcode and badge readers and elastography as part of the system.
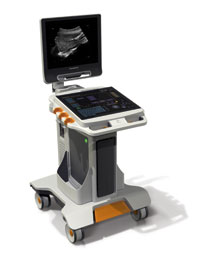
Both the Touch Prime and Touch Prime XE offer a sealed, all-touch control panel that combines the speed and flexibility of a soft user interface with the tactile feedback of traditional keys. Etched marking for primary controls assists the user with easily locating key functions without looking away from the image display monitor.
Both Touch Ultrasound systems deliver these capabilities:
- A sealed panel allows for easy cleaning to help limit the spread of pathogens;
- High-level computing power provides both speed and excellent image quality;
- Easy maneuverability and a small, lightweight footprint make the imaging process faster and easier;
- “Swipe and go” badge log-on saves time and promotes secure access;
- “Smart connect” transducer technology enables easy one-touch selection of the desired transducer;
- Rapid cold boot time of 18 seconds can further help enhance productivity, with no need for standby mode or battery backup; and
- Easy cart adjustments allow sonographers to position the system where it is most comfortable to help reduce the risk of repetitive stress injuries.
The design team for Carestream’s family of Touch Ultrasound systems gathered input from ultrasound professionals around the world to select and refine the capabilities of this new platform, which is supported by Carestream’s global service network.
Carestream’s Touch Prime and Touch Prime XE are expected to ship in the fourth quarter of 2015. Carestream’s family of ultrasound products will include additional systems to address the needs of medical professionals and markets throughout the world.